1. Describe the Fluid Status of This Patient. How Do You Know?
- Debate
- Open Access
- Published:
Fluid overload in the ICU: evaluation and direction
BMC Nephrology volume 17, Article number:109 (2016) Cite this commodity
Abstruse
Groundwork
Fluid overload is frequently found in acute kidney injury patients in critical care units. Recent studies have shown the relationship of fluid overload with agin outcomes; hence, manage and optimization of fluid balance becomes a primal component of the management of critically sick patients.
Discussion
In critically ill patients, in order to restore cardiac output, systemic blood pressure and renal perfusion an adequate fluid resuscitation is essential. Achieving an appropriate level of volume management requires cognition of the underlying pathophysiology, evaluation of book condition, and selection of appropriate solution for volume repletion, and maintenance and modulation of the tissue perfusion. Numerous recent studies accept established a correlation between fluid overload and mortality in critically sick patients. Fluid overload recognition and assessment requires an authentic documentation of intakes and outputs; even so, there is a broad difference in how it is evaluated, reviewed and utilized. Accurate book condition evaluation is essential for appropriate therapy since errors of volume evaluation can result in either in lack of essential treatment or unnecessary fluid administration, and both scenarios are associated with increased mortality. There are several methods to evaluate fluid status; yet, most of the tests currently used are fairly inaccurate. Diuretics, especially loop diuretics, remain a valid therapeutic alternative. Fluid overload refractory to medical therapy requires the application of extracorporeal therapies.
Summary
In critically ill patients, fluid overload is related to increased mortality and also lead to several complications similar pulmonary edema, cardiac failure, delayed wound healing, tissue breakdown, and dumb bowel function. Therefore, the evaluation of volume status is crucial in the early management of critically ill patients. Diuretics are frequently used as an initial therapy; notwithstanding, due to their limited effectiveness the apply of continuous renal replacement techniques are often required for fluid overload treatment. Successful fluid overload treatment depends on precise assessment of individual volume status, understanding the principles of fluid management with ultrafiltration, and clear treatment goals.
Background
Fluid overload is oftentimes institute in critically sick patients with astute kidney injury (AKI). Increasing fluid overload should not merely be considered an expected consequence of fluid resuscitation or astringent AKI, information technology should be seen as a probably mediator of adverse outcomes. In critically sick patients, recent studies have highlighted the function of fluid overload on agin outcomes [i]. Observational studies in pediatric patients who required continuous renal replacement therapy (CRRT) have shown an association between fluid overload and bloodshed [ii–iv]. Restrictive fluid direction strategies are beneficial during acute respiratory distress syndrome and post-obit major surgery since they reduce the duration of mechanical ventilation and the charge per unit of cardiopulmonary complications [5, 6]. In concert with these information, the control and optimization of fluid remainder is a key chemical element of critically sick patients direction, since inadequate fluid removal is associated with peripheral edema and pulmonary edema, which tin retard weaning from mechanical ventilation, or compromise wound healing. Nosotros will focus on the evaluation and management of fluid overload in the intensive care unit (ICU).
Discussion
The role of fluid therapy in the evolution of fluid overload
In critically ill patients, adequate fluid resuscitation is essential to the restoration of cardiac output, systemic blood pressure and renal perfusion in patients with cardiogenic or septic daze [7, 8]. Prompt and adequate treatment with intravenous solutions can also prevent or limit subsequent AKI [9]. Achieving an appropriate level of volume direction requires cognition of the underlying pathophysiology, evaluation of volume status, selection of appropriate solution for volume repletion, and maintenance and modulation of the tissue perfusion [10].
The administration of crystalloids solutions that are recommend for the initial direction of patients with or at risk of AKI, and also in patients with sepsis expands the extracellular compartment, but over fourth dimension since critically ill patients take a increased capillary leak intravenous solutions will leave the circulation and distribute in the extracellular volume leading to edema and to fluid overload. These results in dumb oxygen and metabolite diffusion, distorted tissue architecture, obstacle of capillary blood menstruation and lymphatic drainage, and disturbed cell to jail cell interactions that may then contribute to progressive organ dysfunction (Table i). These effects are prominent in encapsulated organs (liver and kidneys) [11–13]. Fluid overload is not only a outcome of fluid therapy but also occurs during severe sepsis secondary to the release of complement factors, cytokines and prostaglandin products and altered organ microcirculation [fourteen]. In this context, edema is attributed to a combination of increased capillary permeability to proteins and increased net trans-capillary hydrostatic pressure level through reduced pre-capillary vasoconstriction [fifteen].
Fluid overload and outcomes
Several observational studies have demonstrated a correlation between fluid overload and mortality in critically ill patients with acute respiratory distress syndrome, acute lung injury, sepsis, and AKI. Bouchard et al., have shown that patients with fluid overload defined every bit an increase in torso weight of over 10 % had significantly more respiratory failure, need of mechanical ventilation, and more than sepsis. After adjusting for severity of illness, AKI patients with fluid overload had increased xxx mean solar day and 60 day mortality. Amongst survivors, AKI patients who required renal replacement therapy had a significantly lower level of fluid accumulation at initiation of dialysis and at dialysis cessation than not-survivors. Renal recovery was significantly lower in patients with fluid overload [ane]. In children, a multicenter prospective study found that the pct of fluid aggregating at initiation of CRRT was significantly lower in the survivors (fourteen.2 % ±15.9 % vs. 25.iv % ±32.ix %, P = 0.03) [3].
Lungs are 1 of the organs in which adverse effects of fluid overload are most evident, which can lead to acute pulmonary edema or acute respiratory distress syndrome [xvi]. Several studies have provided evidence associating positive fluid balances with poorer respiratory outcomes. In one of these studies, septic shock patients with acute lung injury who received conservative fluid direction subsequently initial fluid resuscitation had lower in-hospital mortality [17]. In some other study, Wiedemann et al. randomized 1000 patients to either a bourgeois or to a liberal strategy of fluid management. Patients randomized to the bourgeois fluid strategy had lower cumulative fluid residue, improved oxygenation index and lung injury score, increased number of ventilator-free days, and reduction in the length of ICU stay. It is worth to mention that the conservative fluid direction strategy did not increase the incidence or prevalence of shock during the study or the demand for renal replacement therapies [five]. Finally, in the Vasopressin in Septic Shock Trial (VASST) written report authors found that college positive fluid balance correlated significantly with increased mortality with the highest mortality rate observed in those with central venous pressure >12 mmHg [18].
Fluid overload recognition and assessment
Fluid overload recognition and assessment in critically ill patients requires an accurate documentation of intakes and outputs; all the same, there is a broad variation in how this information is recorded, reviewed and utilized. Mehta RL and Bouchard J proposed some useful definitions to help us to standardize the approach and facilitated comparisons [10]:
- 1.
Daily fluid residual : daily divergence in all intakes and all outputs, which ofttimes does not include insensible losses.
- two.
Cumulative fluid residual : sum of each 24-hour interval fluid balance over a period of time.
- 3.
Fluid overload : usually implies a degree of pulmonary edema or peripheral edema.
- 4.
Fluid accumulation : positive fluid balance, with or without linked fluid overload.
- 5.
Pct of fluid overload adjusted for body weight : cumulative fluid balance that is expressed as a pct. A cutoff of ≥10 % has been associated with increased mortality. Fluid overload percentage can exist calculated using the following formula [19]:
$$ \%\ \mathbf{Fluid}\ \mathbf{overload}=\left(\left(\mathrm{total}\ \mathrm{fluid}\ \mathrm{in}-\mathrm{total}\ \mathrm{fluid}\ \mathrm{out}\right)/\mathrm{access}\ \mathrm{torso}\ \mathrm{weight}\times 100\right) $$
Fluid status assessment
Accurate volume status evaluation is essential for advisable therapy as inadequate assessment of volume condition tin result in not providing necessary handling or in the administration of unneeded therapy, both associated with increased mortality. There are several methods to evaluate fluid status; all the same, most of the tests currently used are fairly inaccurate. We will describe some of these methods.
-
History and concrete test:
The usefulness of medical history, symptoms, and signs along with routine diagnostic studies (chest radiograph, electrocardiogram, and serum B-type natriuretic peptide (BNP)) that differentiate heart failure from other causes of dyspnea in the emergency department were evaluated in a meta-analysis. Many features increased the probability of middle failure, with the all-time feature for each category beingness the presence of past history of heart failure (positive LR = 5.viii; 95 % CI, four.1–8.0); paroxysmal nocturnal dyspnea (positive LR = 2.half dozen; 95 % CI, 1.5–4.five); 3rd heart audio gallop (positive LR = 11; 95 % CI, 4.9–25.0); breast radiograph showing pulmonary venous congestion (positive LR = 12.0; 95 % CI, 6.eight–21.0); and electrocardiogram showing atrial fibrillation (positive LR = 3.8; 95 % CI, ane.7–viii.8). A low serum BNP proved to be the about useful examination (serum BNP <100 pg/mL; negative LR = 0.xi; 95 % CI, 0.07–0.16) [20].
Importantly, signs like pulmonary rales, lower extremity edema, and jugular venous distention accept significant limits for assessing fluid overload. In that location are some studies that accept correlated these sings during physical examination and invasive measures (eastward.yard., pulmonary catheter wedge pressure (PCWP)). Butman et al. [21] found that the presence of jugular venous distension, at rest or inducible, had a sensitivity (81 %), and a specificity (80 %) for elevation of the pulmonary capillary wedge force per unit area (≥18 mmHg). Using hepato-jugular reflux and Valsalva maneuvers, Marantz et al. showed that these maneuvers were valid in the diagnosis of congestive center failure in acutely dyspneic patients, with depression a sensitivity (24 %) and a high specificity (94 %) [22].
On the other mitt, in a prospective report, concrete signs of fluid overload were compared with hemodynamic measurements in fifty patients with known chronic heart failure. Sings like rales, edema, and elevated mean jugular venous pressures were absent in xviii of 43 patients with pulmonary capillary wedge pressures ≥22 mmHg. The combination of these signs had a sensitivity of 58 % and specificity of 100 % [23].
-
Chest radiography
Chest x-ray has been one of the nearly used tests to evaluate for hypervolemia. Radiographic sings of book overload include dilated upper lobe vessels, cardiomegaly, interstitial edema, enlarged pulmonary artery, pleural effusion, alveolar edema, prominent superior vena cava, and Kerley lines. However, up to 20 % of patients diagnosed with centre failure had negative chest radiographs at initial emergency department evaluation. Additionally, these radiographic sings can be minimal in patients with late-phase middle failure [24].
In patients with congestive heart failure, radiographic signs had poor predictive value for identifying patients with PCWP values ≥thirty mmHg where radiographic pulmonary congestion was absent in 39 % of patients [25].
The Ten-ray technique and the clinical status of patient bear upon radiographic operation for detecting volume overload. Portably chest X-ray, reduce the sensitivity of findings of volume overload [26], and pleural effusions can be missed if the movie is performed supine. With intubated patients and patients with pleural effusions, the sensitivity, specificity, and accuracy of supine breast X-ray was reported to exist as low as 60 %, 70 %, and 67 % respectively [27]. Conversely, the frequency of volume overload findings in the chest 10-ray increased with the severity of fluid overload such as severe heart failure [28].
-
Natriuretic peptides
Loftier levels of BNP tin be found with volume overload; however, some weather condition like myocardial infraction and pulmonary embolism can crusade elevated levels of BNP. Other conditions that have to exist taken into business relationship when evaluating BNP levels are obesity, associated with lower BNP levels and renal failure, associated with high BNP levels. Patients with heart failure who have elevated base-line levels of BNP.
The greatest utility of BNP levels is in the absenteeism of elevation, since low BNP levels take a loftier negative predictive value for excluding middle failure diagnosis. On the other hand, high BNP levels tin can exist not-specific for volume overload [26].
-
Bioimpedance vector assay
Bioelectrical impedance analysis is a commonly used method for estimating torso composition, specifically detecting soft tissue hydration with a 2–3 % measurement error. It is a noninvasive, inexpensive and highly versatile test that transforms electrical properties of tissues into clinical information [29]. Bioimpedance vector analysis (BIVA) measures whole body fluid book and is based on patterns of the resistance-reactance graph, relating trunk impedance to torso hydration [29]. Clinical information on hydration is obtained through patterns of vector distribution with respect to the good for you population of the aforementioned race, sex, class of torso mass alphabetize, and age. Changes in tissue hydration status below 500 ml are detected and ranked. BIVA was examined as an indicator of fluid status compared to central venous pressure (CVP) in 121 critically ill patients [30]. In this written report patients were classified in three groups co-ordinate to their CVP value: low (0 to three mmHg); medium (4 to 12 mmHg); and high (13 to xx mmHg). The agreement between BIVA and central venous pressure level indications was skillful in the high CVP group, moderate in the medium CVP grouping, and poor in depression CVP group. The combined evaluation of peripheral tissue hydration (BIVA) and primal filling force per unit area (CVP) could provide a useful clinical assessment instrument in the planning of fluid therapy in critically sick patients, particularly in those with low CVP [31].
-
Thoracic ultrasound
Sonographic artifacts known as B-lines that suggest thickened interstitial or fluid-filled alveoli can be detected using thoracic ultrasound (Fig. 1). PCWP and fluid accumulation in lungs have been correlated with the presence of B-lines ("comet-tail images") in patients with congestive heart failure [32]. Agricola et al., used thoracic ultrasound to discover "comet-tail images" and obtained an individual patient comet-tail image score by summing the number of B-lines in each of the scanned spaces assessed (right and left hemi thorax, from second to fourth intercostals' space, from para-sternal to mid-axillary line); authors found significant positive linear correlations betwixt comet-tail images score and actress-vascular lung water adamant by the PiCCO System, between comet score and PCWP, and between comet-tail images score and radiologic sings of fluid overload in the lungs [33].
Fig. ane 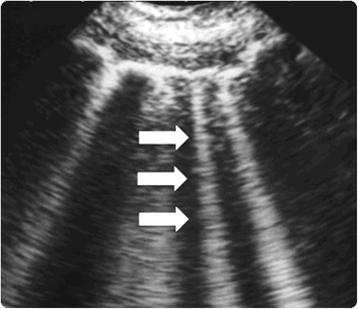
Lung comet tail image. 'B lines' as well known as comet-tail images are a marker of pulmonary edema. In the presence of extravascular lung water the reflection of the ultrasound axle on the sub-pleural interlobular septa thickened by edema creates comet-tail reverberation artifacts. The ultrasound advent is of a vertical, discrete, hyperechogenic paradigm that arises from the pleural line and extends to the lesser of the screen moving synchronously with the respiration (white arrows)
-
Vena cava diameter ultrasound
The measurement of the inferior vena cava (IVC) diameter tin too exist use to assess book status. Normal diameter of IVC is ane.5 to ii.5 cm (measured 3 cm from the right atrium); volume depletion is considered with an IVC diameter <1.v cm while an IVC diameter >2.5 cm suggests volume overload.
In an observational written report on blood donors, Lyon et al. evaluated the inferior vena cava diameter (IVCd) during inspiration (IVCdi) and during expiration (IVCde), before and after claret donation of 450 mL. Significant differences were establish between the IVCde before and later claret donation and between IVCdi before and after donation (five.v mm and five.16 mm, respectively) [34]. In patients treated for hypovolemia, Zengin et al. evaluated the IVC and right ventricle (RVd) diameters and diameter changes with the diameters and diameter changes of salubrious volunteers. The IVCd was measured ultrasonographically past Thou-manner in the subxiphoid area and the RVd was measured in the 3rd and fourth intercostals spaces before and after fluid resuscitation. As compare with healthy volunteers boilerplate diameters in hypovolemic patients of the IVC during inspiration and expiration, and right ventricule diameter were significantly lower. After fluid resuscitation, there was a significant increase in mean IVC diameters during inspiration and expiration as well every bit in the right ventricule bore [35]. Bedside inferior vena cava bore and right ventricule bore evaluation could be a applied noninvasive instrument for fluid status interpretation and for evaluating the response to fluid therapy in critically ill patients.
Fluid overload management
Diuretic therapy
Diuretics, peculiarly loop diuretics, remain a valid therapeutic alternative for relieving symptoms and improving pathophysiological states of fluid overload such equally congestive heart failure and in patients with AKI. At this time, in that location is no evidence that favors ultrafiltration over diuretic use in book overload patients with or without AKI in terms of less progression of AKI, improved clinical outcomes or reduce incidence of AKI [36]. Despite that more patients adult AKI during diuretic treatment, numerous studies accept demonstrated that more aggressive use of loop diuretics to achieve greater volume removal is associated with improved outcomes (Table 2) [37–40].
What should be the goal of urine output when using diuretics to manage fluid overload? Some empirical observations have shown that a urine output of three–4 ml/kg/h rarely causes intravascular book depletion as capillary refill can see such rates in near all patients [41]. Diuretics could be either administered past bolus or using a continuous infusion. In that location has been controversy about which of these strategies is better; some authors advocate that diuretic infusion is superior to boluses since urinary output could be maintain easily [41]. In one study diuretic infusion was associated with greater diuresis and this was achieved with a lesser dose [42]; infusion was also associated with fewer adverse events such as worsening AKI, hypokalemia, and ototoxicity. However, in the DOSE-AHF(Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure) study, authors plant that patients with acute heart failure may benefit from an initial bolus strategy [43].
Since mutual electrolyte disturbances could be encountered during diuretic therapy, it is of import to monitor electrolytes levels and too to assess acid-based condition. In lodge to avoid hypokalemia, administration of oral potassium it is easy. Measuring urinary potassium concentration and calculating the daily losses of potassium, which crave replacement is a strategy that can be used to estimate daily potassium requirements. Another strategy is the use of potassium-sparing diuretics similar spironolactone. Hypomagnesemia is oft establish during diuretic therapy, magnesium replacement tin can exist achieved either intravenously or orally, typically with 20–30 mmoL per day. Finally in some patients, chloride losses exceed sodium losses and hypochloremic metabolic alkalosis develops; this is usually corrected with the administration of potassium chloride and magnesium chloride.
A contempo comprehensive review take shown that torsemide and bumetanide take more than favorable pharmacokinetic profiles than furosemide, and in the case of torsemide it could exist more efficacious than furosemide in patients with eye failure (decreased mortality, decrease hospitalizations, and improved New York Heart Clan functional classification). In AKI patients, every bit compared with torsemide the use of furosemide was associated with a pregnant improvement in urine output. Moreover, two trials comparing bumetanide with furosemide showed conflicting results [44].
Finally, in patients with AKI the response to furosemide may be reduced due to multiple mechanisms including a reduced tubular secretion of furosemide and blunted response of Na-One thousand-2Cl co-transporters at the loop of Henle [45]. This reduced response to furosemide in AKI patients often requires the utilize of college doses that may increase the risk of ototoxicity, especially as the clearance of furosemide is severely reduced in AKI. High doses of furosemide may also effect in myocardial dysfunction secondary to furosemide induced vasoconstriction [46].
Extracorporeal therapies
Fluid overload refractory to medical therapy requires the utilise of extracorporeal therapies such as continuous renal replacement therapies since critically ill patients frequently show hemodynamic instability and/or multiple organ dysfunctions. Authentic management of fluid balance becomes obligatory with the ultimate goal of improving pulmonary gas exchange and organ perfusion while maintaining stable hemodynamic parameters. The optimal renal replacement therapy for patients with AKI and fluid overload has not been defined yet and there is still an ongoing contend. Choice of the initial modality needs to be based on the availability of resource, local expertise; the individual needs of the patients, and finally on patient'southward hemodynamic status.
In patients with fluid overload, CRRT provides a slower fluid removal over intermittent hemodialysis (IHD) resulting in more hemodynamic stability and improve fluid balance control, other advantages of CRRT over IHD include: a slower command of solute concentration avoiding large fluctuations and fluid shifts, which reduce the take a chance of cerebral edema, the smashing flexibility in terms of treatment aligning to patient's needs at someday, and finally CRRT allows to perform the treatment with relatively simple and user friendly machines [47]. Some large observational studies have suggested that CRRT is an independent predictor of renal recovery amid survivors [48–50].
In the absenteeism of definite data to support the use of item blazon of renal replacement therapy, one should consider CRRT and IHD as complementary therapies. Therefore, during the treatment of critically ill patients with AKI and fluid overload transitions between CRRT and IHD are frequent, and are frequently driven by patients' hemodynamic condition.
Slow continuous ultrafiltration (SCUF) is a type of continuous renal replacement therapy that is usually performed with low blood flow rates (fifty to 100 ml/min), and ultrafiltration rates between 100 and 300 ml/h according to fluid residuum necessities. Relatively small surface-area filters can be employed with reduced heparin doses since depression ultrafiltration and blood flow rates are required, [51].
Continuous veno-venous hemofiltration (CVVH) is another CRRT technique that allows meticulous, minute-to-minute command of fluid balance past providing continuous fluid, electrolyte, and toxin clearance.
The prescription of CRRT related fluid management and its integration into overall patient fluid management could exist improved by using a specific club chart for the machine fluid balance every bit shown on Table three. Auto fluid balance refers to the total balance over 24-h period of fluids administered past the CRRT machine (dialysate or replacement fluid or both depending on the technique) and fluids removed past the CRRT car (spent dialysate or ultrafiltrate or both depending on the technique). This set up volition aid to reach the planned hourly fluid remainder as shown on Table iii and Fig. 2.
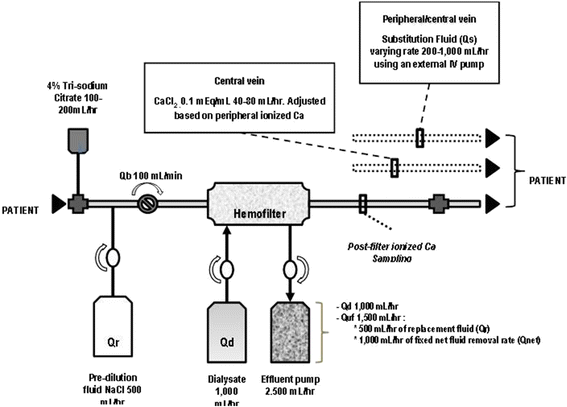
Excursion set at University of California San Diego, Medical Heart. The mean infusion charge per unit of tri-sodium citrate was 180 ml/h and blood menstruation rate (Qb) was set at 100 ml/min. Tri-sodium citrate was added at the arterial catheter port with ionized calcium levels been measured postal service-filter. Mail service-filter ionized calcium levels were used to suit tri-sodium citrate menstruum rates. Pre-filter BUN value was measured afterwards the infusion of tri-sodium citrate and after pre-dilution replacement fluid (Qr), thus bookkeeping for the pre-dilutional upshot. A fixed ultrafiltration charge per unit (Quf) was used (fix at one thousand ml/h) for achieving fluid balance. A target effluent volume was adjusted by hourly modifying substitution fluid rate (Qs) to achieve a negative, zero, or positive fluid balance. Qb, blood menses rate; Qd, dialysate catamenia rate; Qr, replacement fluid charge per unit; Quf, total ultrafiltration rate; Qnet, net fluid removal charge per unit
The ultimate goal is to preserve tissue perfusion, optimizing fluid remainder past effectively removing fluid without compromising the effective circulating fluid volume; therefore, meticulous monitoring of fluid balance is disquisitional for all patients [52].
Another pick for treating patients with fluid overload are the new smaller and more portable devices similar the Aquadex FlexFlow Organisation (Baxter Healthcare). In patients with centre failure, Costanzo et al. compare adjustable ultrafiltration using a minor ultrafiltration device to the use of intravenous loop diuretics. The authors found a tendency to longer time to recurrence of middle failure within 90 days consequence after infirmary discharge in patients treated with the ultrafiltration device, and fewer middle failure and cardiovascular events. Changes in renal function and the 90-day bloodshed were like in both groups. However, more patients who were randomized to adjustable ultrafiltration experienced an agin effect of special interest (p = 0.018) and a serious study production-related adverse events (p = 0.026) [53].
Conclusions
Several complications like congestive heart failure, pulmonary edema, delayed wound healing, tissue breakup, and impaired bowel office are associated with fluid overload. Fluid overload has also been related to increased mortality. The optimal assessment of volume status in critically ill patients is of vital importance particularly during the early on direction of these patients. One key aspect of fluid overload management is to maintain hemodynamic stability and optimize organ function. Loop diuretics are frequently used as the initial therapy to treat critically sick patients with fluid overload; nevertheless, diuretics have express effectiveness due to several factors such as underlying acute kidney injury that contribute to diuretic resistance. Renal replacement therapies are often required for optimal volume management in critically ill patients with fluid overload. In this setting, successful volume management depends on an accurate estimation of patients' fluid status, an adequate agreement of the principles of fluid overload treatment with ultrafiltration, and articulate treatment goals.
Abbreviations
AKI, astute kidney injury; BIVA, Bio-impedance vector analysis; BNP, B-type natriuretic peptide; CRRT, continuous renal replacement therapy; CVP, primal venous pressure; CVVH, continuous veno-venous hemofiltration; EVLW, extra-vascular lung water; ICU, intensive care unit; IHD, intermittent hemodialysis; IVC, inferior vena cava; IVCd, inferior vena cava diameter; IVCde, inferior vena cava bore during expiration; IVCdi, inferior vena cava diameter during inspiration; PCWP, pulmonary catheter wedge pressure; RVd, right ventricle diameter; SCUF, slow continuous ultrafiltration
References
-
Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with astute kidney injury. Kidney Int. 2009;76(four):422–7.
-
Goldstein SL, Currier H, Graf C, Cosio CC, Brewer ED, Sachdeva R. Upshot in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309–12.
-
Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005;67(ii):653–eight.
-
Gillespie RS, Seidel Grand, Symons JM. Effect of fluid overload and dose of replacement fluid on survival in hemofiltration. Pediatr Nephrol. 2004;xix(12):1394–9.
-
Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of 2 fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–75.
-
Brandstrup B, Tonnesen H, Beier-Holgersen R, Hjortso Due east, Ording H, Lindorff-Larsen K, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of ii perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg. 2003;238(5):641–8.
-
Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid residuum and acute kidney injury. Nat Rev Nephrol. 2010;6(2):107–15.
-
Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, et al. Outcomes of the Surviving Sepsis Campaign in intensive care units in the U.s.a. and Europe: a prospective cohort written report. Lancet Infect Dis. 2012;12(12):919–24.
-
Kellum JA, Lameire Northward. Kidney Illness Improving Global Outcomes (KDIGO) Working Group. Section 3: Prevention and Treatment of AKI. Kidney Int Suppl (2011). 2012;ii(1):37–68.
-
Mehta RL, Bouchard J. Controversies in acute kidney injury: effects of fluid overload on event. Contrib Nephrol. 2011;174:200–11.
-
Humphrey H, Hall J, Sznajder I, Silverstein Thou, Wood Fifty. Improved survival in ARDS patients associated with a reduction in pulmonary capillary wedge pressure. Breast. 1990;97(5):1176–80.
-
Nisanevich V, Felsenstein I, Almogy Chiliad, Weissman C, Einav S, Matot I. Event of intraoperative fluid management on effect later intraabdominal surgery. Anesthesiology. 2005;103(ane):25–32.
-
Boyle A, Maurer MS, Sobotka PA. Myocellular and interstitial edema and circulating volume expansion as a cause of morbidity and bloodshed in heart failure. J Carte Neglect. 2007;13(2):133–6.
-
Andreucci One thousand, Federico Southward, Andreucci VE. Edema and acute renal failure. Semin Nephrol. 2001;21(3):251–6.
-
Bouchard J, Mehta RL. Fluid balance issues in the critically ill patient. Contrib Nephrol. 2010;164:69–78.
-
Schrier RW, Wang W. Acute renal failure and sepsis. Due north Engl J Med. 2004;351(2):159–69.
-
Murphy CV, Schramm GE, Doherty JA, Reichley RM, Gajic O, Afessa B, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136(1):102–9.
-
Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid residual and elevated central venous pressure are associated with increased bloodshed. Crit Intendance Med. 2011;39(2):259–65.
-
Bagshaw SM, Cruz DN. Fluid overload every bit a biomarker of heart failure and acute kidney injury. Contrib Nephrol. 2010;164:54–68.
-
Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944–56.
-
Butman SM, Ewy GA, Standen JR, Kern KB, Hahn Due east. Bedside cardiovascular examination in patients with astringent chronic eye failure: importance of residue or inducible jugular venous amplification. J Am Coll Cardiol. 1993;22(4):968–74.
-
Marantz PR, Kaplan MC, Alderman MH. Clinical diagnosis of congestive center failure in patients with astute dyspnea. Breast. 1990;97(4):776–81.
-
Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261(six):884–viii.
-
Collins SP, Lindsell CJ, Storrow AB, Abraham WT. Prevalence of negative chest radiography results in the emergency department patient with decompensated center failure. Ann Emerg Med. 2006;47(1):13–8.
-
Chakko South, Woska D, Martinez H, de Marchena East, Futterman 50, Kessler KM, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive eye failure: conflicting results may lead to inappropriate care. Am J Med. 1991;ninety(3):353–9.
-
Peacock WF, Soto KM. Electric current techniques of fluid status assessment. Contrib Nephrol. 2010;164:128–42.
-
Ruskin JA, Gurney JW, Thorsen MK, Goodman LR. Detection of pleural effusions on supine breast radiographs. AJR Am J Roentgenol. 1987;148(4):681–three.
-
Chait A, Cohen HE, Meltzer LE, VanDurme JP. The bedside chest radiograph in the evaluation of incipient center failure. Radiology. 1972;105(3):563–vi.
-
Piccoli A. Patterns of bioelectrical impedance vector assay: learning from electrocardiography and forgetting electric excursion models. Nutrition. 2002;eighteen(half dozen):520–1.
-
Piccoli A, Pittoni G, Facco E, Favaro E, Pillon L. Human relationship between key venous pressure and bioimpedance vector analysis in critically ill patients. Crit Intendance Med. 2000;28(ane):132–seven.
-
Piccoli A. Bioelectric impedance measurement for fluid condition assessment. Contrib Nephrol. 2010;164:143–52.
-
Picano E, Frassi F, Agricola E, Gligorova South, Gargani L, Mottola G. Ultrasound lung comets: a clinically useful sign of extravascular lung water. J Am Soc Echocardiogr. 2006;19(3):356–63.
-
Agricola Eastward, Bove T, Oppizzi M, Marino G, Zangrillo A, Margonato A, et al. "Ultrasound comet-tail images": a mark of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127(5):1690–5.
-
Lyon Thou, Blaivas M, Brannam L. Sonographic measurement of the inferior vena cava equally a marker of blood loss. Am J Emerg Med. 2005;23(ane):45–fifty.
-
Zengin Southward, Al B, Genc Due south, Yildirim C, Ercan S, Dogan M, et al. Role of inferior vena cava and correct ventricular diameter in assessment of volume status: a comparative report: ultrasound and hypovolemia. Am J Emerg Med. 2013;31(5):763–7.
-
Perazella MA, Coca SG. Three feasible strategies to minimize kidney injury in 'incipient AKI'. Nat Rev Nephrol. 2013;nine(8):484–ninety.
-
Mehta RL, Pascual MT, Soroko S, Chertow GM. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288(xx):2547–53.
-
Uchino Due south, Doig GS, Bellomo R, Morimatsu H, Morgera South, Schetz M, et al. Diuretics and bloodshed in acute renal failure. Crit Care Med. 2004;32(eight):1669–77.
-
Cantarovich F, Rangoonwala B, Lorenz H, Verho Grand, Esnault VL. High-dose furosemide for established ARF: a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Am J Kidney Dis. 2004;44(3):402–ix.
-
Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD. Fluid remainder, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol. 2011;half dozen(v):966–73.
-
Bellomo R, Prowle JR, Echeverri JE. Diuretic therapy in fluid-overloaded and heart failure patients. Contrib Nephrol. 2010;164:153–63.
-
Martin SJ, Danziger LH. Continuous infusion of loop diuretics in the critically ill: a review of the literature. Crit Care Med. 1994;22(8):1323–9.
-
Shah RV, McNulty Due south, O'Connor CM, Felker GM, Braunwald E, Givertz MM. Effect of access oral diuretic dose on response to continuous versus bolus intravenous diuretics in acute heart failure: an assay from diuretic optimization strategies in astute eye failure. Am Heart J. 2012;164(6):862–8.
-
Wargo KA, Banta WM. A comprehensive review of the loop diuretics: should furosemide be first line? Ann Pharmacother. 2009;43(eleven):1836–47.
-
Brater DC. Resistance to diuretics: accent on a pharmacological perspective. Drugs. 1981;22(6):477–94.
-
De Vecchis R, Ciccarelli A, Cioppa C. Intermittent intravenous infusion of high-dose loop diuretics and risk for iatrogenic ototoxicity: an unresolved issue from the DOSE study. G Ital Cardiol (Rome). 2012;xiii(x):701–2. author reply two–4.
-
Kellum JA, Lameire N. Kidney Disease Improving Global Outcomes (KDIGO) Working Grouping.Section v: Dialysis Interventions for Treatment of AKI. Kidney Int Suppl (2011). 2012;2(1):89–115.
-
Bong Chiliad, SWING, Granath F, Schon S, Ekbom A, Martling CR. Continuous renal replacement therapy is associated with less chronic renal failure than intermittent haemodialysis after acute renal failure. Intensive Care Med. 2007;33(5):773–fourscore.
-
Jacka MJ, Ivancinova X, Gibney RT. Continuous renal replacement therapy improves renal recovery from astute renal failure. Can J Anaesth. 2005;52(3):327–32.
-
Uchino S, Bellomo R, Kellum JA, Morimatsu H, Morgera S, Schetz MR, et al. Patient and kidney survival by dialysis modality in critically ill patients with acute kidney injury. Int J Artif Organs. 2007;30(four):281–92.
-
Cerda J, Ronco C. Modalities of continuous renal replacement therapy: technical and clinical considerations. Semin Dial. 2009;22(2):114–22.
-
Bouchard J, Mehta RL. Volume management in continuous renal replacement therapy. Semin Dial. 2009;22(2):146–50.
-
Costanzo MR, Negoianu D, Jaski Be, Bart BA, Heywood JT, Anand IS, et al. Aquapheresis Versus Intravenous Diuretics and Hospitalizations for Heart Failure. JACC Middle Neglect. 2016;four(two):95–105.
Acknowledgements
We want to admit Mary Helen Begley who provided writing services for this manuscript.
Funding
No funding was obtained for this written report.
Availability of data and materials
All the information supporting what is draw in the manuscript in contained within.
Authors' contributions
RCDG and RM participated in writing and helped to draft the manuscript. Both authors read and approved the last manuscript.
Authors' information
RCDG: Rolando Claure-Del Granado received his medical caste from Universidad del Valle, Schoolhouse of Medicine (Cochabamba-Republic of bolivia). His postgraduate preparation included a residency in Internal Medicine and a Fellowship in Nephrology at the Instituto Nacional de Ciencias Médicas y Nutrición "Salvador Zubirán" (Mexico Urban center-Mexico). He was a Inquiry Fellow at the Medicine Department—Partition of Nephrology of the Academy of California San Diego, from 2009 to 2011. Post-obit his fellowship he joined the faculty of Universidad Mayor de San Simon, Schoolhouse of Medicine, Cochabamba-Bolivia; every bit a Professor of Medicine. He is a Clinical Research Investigator at the Biomedical Research Establish (IIBISMED) of the Universidad Mayor de San Simón Schoolhouse of Medicine, Cochabamba-Bolivia. He is a member of the Acute Kidney Injury Committee of the Latin-American Order of Nephrology and Hypertension. He is the Chair of the International Order of Nephrology—Young Nephrologists Committee (ISN-YNC). Dr. Claure-Del Granado'due south enquiry interests include extracorporeal therapies for AKI, the assessment of acid–base metabolic disorders, the report of biomarkers in AKI, and the role of immunity in AKI.
RM: Dr. Mehta received the M.B.B.S. degree (1976) from the Government Medical School in Amritsar, Bharat, and the Chiliad.D. (1979) and D.M. (1981) degrees from the Mail service Graduate Institute of Medical Pedagogy and Research in Chandigarh, India. He afterward completed a nephrology fellowship at the Academy of Rochester in Rochester New York and obtained his boards in internal medicine (1986) and Nephrology (1988). He is a Professor of Medicine in the Division of Nephrology and Associate Chair for Clinical Affairs in the Section of Medicine at the University of California, San Diego (UCSD) where he directs the Clinical nephrology and dialysis programs. He is also the Chair of the International Order of Nephrology 0by25 initiative, founding member of the Acute Dialysis Quality Initiative. His research interests are in the field of acute kidney injury (AKI) and he has directed several clinical studies in the management of patients with AKI including comparing unlike types of renal replacement therapies, conducting several large multicenter observational studies of AKI, evaluating unlike predictive models for outcomes in AKI, investigating the role of cytokine removal by dialysis membranes in sepsis and AKI, and evaluating techniques for determining the amount of backlog fluid in dialysis patients.
Competing interests
The authors declare that they accept no competing interests.
Consent for publication
Non applicable.
Ideals approval and consent to participate
The local ethics committee does non request formal ethics committee approving when a review manuscript is submitted, this is just required when clinical or basic research is washed.
Author data
Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution four.0 International License (http://creativecommons.org/licenses/by/iv.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you lot give appropriate credit to the original author(due south) and the source, provide a link to the Artistic Commons license, and signal if changes were fabricated. The Artistic Eatables Public Domain Dedication waiver (http://creativecommons.org/publicdomain/nix/one.0/) applies to the data made available in this commodity, unless otherwise stated.
Reprints and Permissions
Nigh this article
Cite this article
Claure-Del Granado, R., Mehta, R.L. Fluid overload in the ICU: evaluation and management. BMC Nephrol 17, 109 (2016). https://doi.org/10.1186/s12882-016-0323-6
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1186/s12882-016-0323-6
Keywords
- Fluid overload
- Acute kidney injury
- Diuretics
- Continuous renal replacement therapies
Source: https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-016-0323-6
0 Response to "1. Describe the Fluid Status of This Patient. How Do You Know?"
Post a Comment